| Stages of Development Summary | Child Development Stages |
| Human Development (textbook) | Fetal Psychology |
How Does a Single Cell Become a Whole Body?
We all begin existence as the simplest thing you can imagine if you’re trying to picture life: a single cell--nothing, in other words, but a tiny, spherical bag of proteins. In fact, the average one-celled amoeba looks far perkier under a microscope than a fertilized human egg. Yet that humble and nondescript appearance is highly deceptive. The egg, after all, boasts dazzling prospects, a future of truly dizzying--and until very recently, utterly baffling--complexity.
When an amoeba divides, all you get is more amoebas. But when a human egg splits--first into 2, then 4, then 8, 16, 32, 64, and so on--it embarks on a venture that will, over the next nine months, produce descendant cells with a huge variety of shapes and functions: bone cells, nerve cells, red and white blood cells; the cells of the eyes, fingernails, stomach, and skin.
Consider, for example, your body’s crowning glory, your head. How did the bone cells in your skull know enough to marshal themselves into a dome, while those in your jaw formed a trap-shaped mandible? And how did those on your left side arrange themselves in a mirror image of your right, and how were holes left in just the right places for your eyes? Come to think of it, how did your head wind up at the top of your body, nodding sagely at the end of your neck and spine? And that’s only the beginning.
How did that bloblike egg give rise to, say, nerve cells in your fingertips with long filaments to cable signals back toward the spine? What informed them to relay their signals to intermediary nerve cells there by zapping them with chemicals? What told those cells’ filaments to twine themselves into a cord inside your spine, levitate toward your skull, and send messages into the folds of your brain for interpretation?…
It’s easy now to picture how an aspiring fertilized egg
ultimatel turns
into a frog or a fly.
The egg comes equipped with different proteins in its different zones. As it divides, the daughter cells are directed--via their developmental genes--down different paths, depending on what part of the egg they took their substance from. It’s as if a unique protein in one section of the
egg tells all the cells formed
there, You be a front
cell, or You’re a
back cell.
Then, as cell division progresses and more and more cells populate the embryo, they begin chattering chemically to each other, specifying more and more complicated information about shape, function, and position. A cell destined to form a foot, for example, might send a message to a neighboring cell directing its descendants to switch on genes that direct the cells outward and shape them into a leg.
What’s more, as embryos develop, their tissues shift around in relation to one another. That means cells can receive new signals as new tissues move within their protein-signaling range. It’s a bit like biological astrology: as tissues slowly cycle through the embryo, they can fall under the sway of new influences, and their fates swerve accordingly.
In a complex creature like a human, this process can take some strange turns. Take the case of the lens of the eye. In the 1960s, research on amphibians showed that the highly specialized cells that form the lens acquire their distinctive shape only after a series of elaborate migrations. Their earliest distinct identity is as skin cells, positioned in the embryo above a group of cells destined to become the pharynx. But gradually some future heart tissue drifts within range, and it sends another series of developmental cues vital to lens formation.
Only later do the future lens cells come into contact with anything you’d intuitively connect with an eye: the maturing retina, which apparently sends the final barrage of messages that complete the transformation of these cells into a lens. (As it happens, some of the cells in the lens of your eye are the very same cells that were laid down shortly after you were a fertilized egg. They were never replaced. As you read this page, you are, so to speak, looking through your own embryo.)
You can see the whole developmental megillah--from egg to body-- neatly illustrated in the Model T organism of genetic research: Drosophila, the common fruit fly.
Shortly after fertilization the cells of the future fly grub begin making their fateful choices. From the mother, the egg inherits instructions to make a protein called bicoid, which spreads through the egg in a smooth gradient, concentrated heavily at the front of the egg and gradually thinning out toward the rear. The high density of bicoid clearly marks the front of the embryo; it also trips a class of genes called gap genes that further define the thorax and abdomen. This takes about two and a half hours and amounts to a preliminary laying out of the construction site--determining what’s fore and what’s aft.
Then the next step begins. Triggered by gap genes, another set of genes (including one named hairy) causes the embryo to display a zebralike pattern of seven stripes. That in turn sets the stage for the division of the embryo into 14 segments, under the auspices of a gene named engrailed. The fore-and-aft theme, so far a sweeping motif affecting the whole embryo, is replayed in each of the 14 segments that now appear, and a fascinating collaboration commences between engrailed and another gene, called wingless…
https://www.discovermagazine.com/mind/how-does-a-single-cell-become-a-whole-body
Human embryonic development - Wikipedia
The beginning of the cleavage process is marked when the zygote divides through mitosis into two cells. This mitosis continues and the first two cells divide into four cells, then into eight cells and so on. Each division takes from 12 to 24 hours. The zygote is large compared to any other cell and undergoes cleavage without any overall increase in size. This means that with each successive subdivision, the ratio of nuclear to cytoplasmic material increases. Initially the dividing cells, called blastomeres (blastos Greek for sprout), are undifferentiated and aggregated into a sphere enclosed within the membrane of glycoproteins (termed the zona pellucida) of the ovum. When eight blastomeres have formed they begin to develop gap junctions, enabling them to develop in an integrated way and co-ordinate their response to physiological signals and environmental cues.
When the cells number around sixteen the solid sphere of cells within the zona pellucida is referred to as a morula[6] At this stage the cells start to bind firmly together in a process called compaction, and cleavage continues as cellular differentiation.
Blastulation
Cleavage itself is the first stage in blastulation, the process of forming the blastocyst. Cells differentiate into an outer layer of cells (collectively called the trophoblast) and an inner cell mass. With further compaction the individual outer blastomeres, the trophoblasts, become indistinguishable. They are still enclosed within the zona pellucida. This compaction serves to make the structure watertight, containing the fluid that the cells will later secrete. The inner mass of cells differentiate to become embryoblasts and polarise at one end. They close together and form gap junctions, which facilitate cellular communication. This polarisation leaves a cavity, the blastocoel, creating a structure that is now termed the blastocyst. (In animals other than mammals, this is called the blastula.) The trophoblasts secrete fluid into the blastocoel. The resulting increase in size of the blastocyst causes it to hatch through the zona pellucida, which then disintegrates.
The inner cell mass will give rise to the pre-embryo, the amnion, yolk sac and allantois, while the fetal part of the placenta will form from the outer trophoblast layer. The embryo plus its membranes is called the conceptus, and by this stage the conceptus has reached the uterus.
- The zona pellucida ultimately disappears completely, and the now exposed cells of the trophoblast allow the blastocyst to attach itself to the endometrium, where it will implant.
- The formation of the hypoblast and epiblast, which are the two main layers of the bilaminar germ disc, occurs at the beginning of the second week.
- Either the embryoblast or the trophoblast will turn into two sub-layers. The inner cells will turn into the hypoblast layer, which will surround the other layer, called the epiblast, and these layers will form the embryonic disc that will develop into the embryo.
- The trophoblast will also develop two sub-layers: the cytotrophoblast, which is in front of the syncytiotrophoblast, which in turn lies within the endometrium.
- Next, another layer called the exocoelomic membrane or Heuser’s membrane will appear and surround the cytotrophoblast, as well as the primitive yolk sac.
- The syncytiotrophoblast will grow and will enter a phase called lacunar stage, in which some vacuoles will appear and be filled by blood in the following days.
- The development of the yolk sac starts with the hypoblastic flat cells that form the exocoelomic membrane, which will coat the inner part of the cytotrophoblast to form the primitive yolk sac.
- An erosion of the endothelial lining of the maternal capillaries by the syncytiotrophoblastic cells of the sinusoids will form where the blood will begin to penetrate and flow through the trophoblast to give rise to the uteroplacental circulation.
- Subsequently new cells derived from yolk sac will be established between trophoblast and exocelomic membrane and will give rise to extra-embryonic mesoderm, which will form the chorionic cavity.
At the end of the second week of development, some cells of the trophoblast penetrate and form rounded columns into the syncytiotrophoblast. These columns are known as primary villi. At the same time, other migrating cells form into the exocelomic cavity a new cavity named the secondary or definitive yolk sac, smaller than the primitive yolk sac.
Let’s Start at the Very Beginning
- Step 1: a zygote is the single cell formed when an egg and a sperm cell fuse; the fusion is known as fertilization
- Step 2: the first 12-to 24-hours after a zygote is formed are spent in cleavage – very rapid cell division
Blastulation and Cell Differentiation
- Step 3: during blastulation, the mass of cells forms a hollow ball
- Step 4: cells begin to differentiate, and form cavities
Making Tubes
- Step 5: During gastrulation the three germ layers form; the cell mass is now known as a gastrula
- Step 5a: The primitive streak forms
- Step 6: The notochord is formed
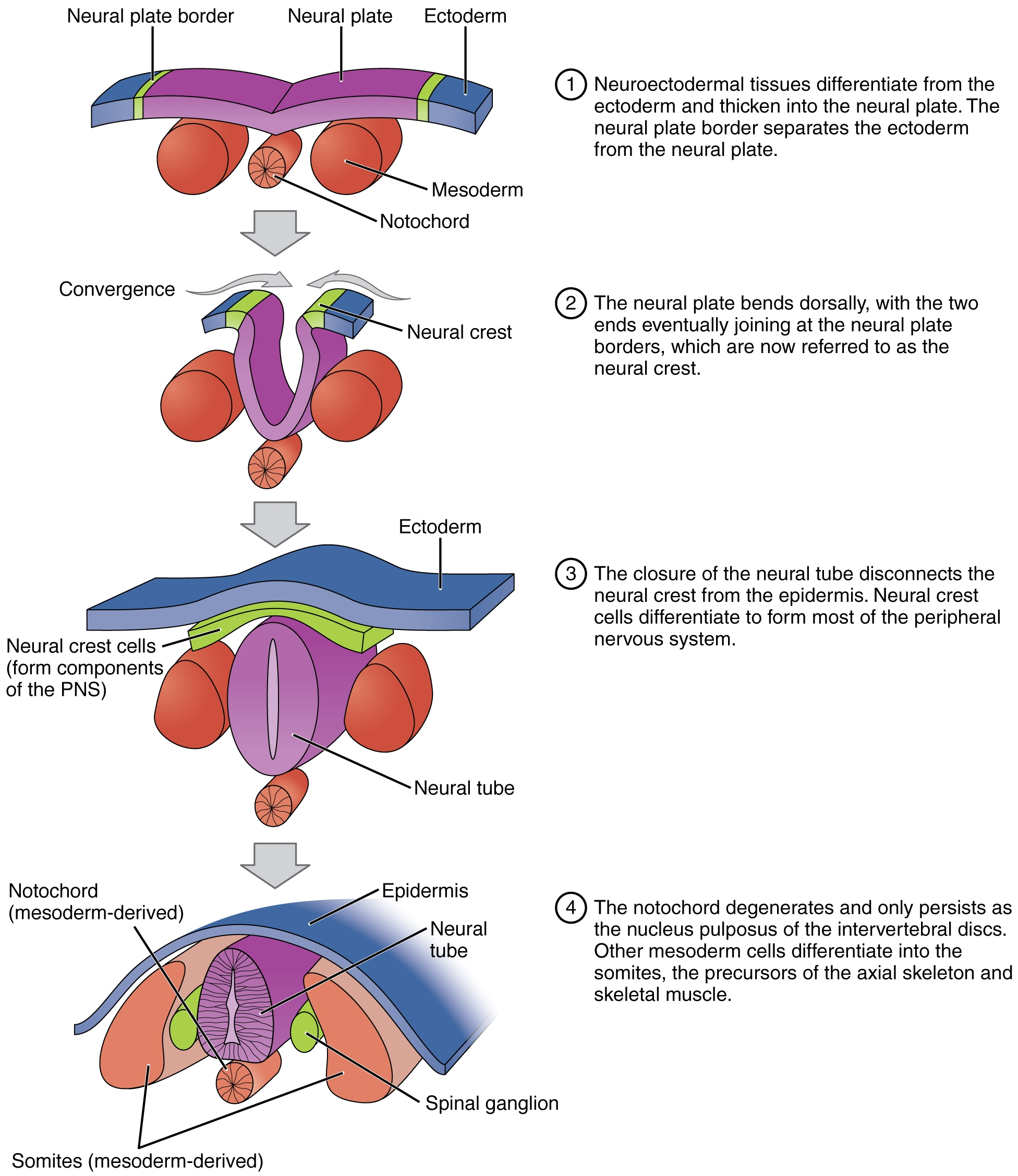
Neurulation
- Step 6: Tubes form, making a neurula
- Step 6a: The notochord induces the formation of the neural plate
- Step 6b: The neural plate folds in on itself to make the neural tube and neural crest
- Step 7: The mesoderm has five distinct categories
Consider the following
How Turning Particular Genes On or Off, in the Same Genome in Each Cell, Allows the Creation of 100s of Unique Cell Types
Gene Expression Regulates Cell Differentiation - How is it that your body with all of its specialized organs developed from a single cell? Gene expression patterns and their timing regulate cell differentiation.
All of the cells within a complex multicellular organism such as a human being contain the same DNA; however, the body of such an organism is clearly composed of many different types of cells. What, then, makes a liver cell different from a skin or muscle cell? The answer lies in the way each cell deploys its genome. In other words, the particular combination of genes that are turned on (expressed) or turned off (repressed) dictates cellular morphology (shape) and function.
This process of gene expression is regulated by cues from both within and outside cells, and the interplay between these cues and the genome affects essentially all processes that occur during embryonic development and adult life.
http://www.nature.com/scitable/topicpage/gene-expression-regulates-cell-differentiation-931
All the parts of your body are made up of cells. There is no such thing as a typical cell. Your body has many different kinds of cells. Though they might look different under a microscope, most cells have chemical and structural features in common. In humans, there are about 200 different types of cells, and within these cells there are about 20 different types of structures or organelles.
http://sciencenetlinks.com/student-teacher-sheets/cells-your-body/
From One Genome, Many Types of Cells. But How? One of the enduring mysteries of biology is that a variety of specialized cells collaborate in building a body, yet all have an identical genome. Somehow each of the 200 different kinds of cells in the human body - in the brain, liver, bone, heart and many other structures - must be reading off a different set of the hereditary instructions written into the DNA.
A Cell's Many Faces - The Epigenome Guides Cells to Their Specialized Roles - The system is something like a play in which all the actors have the same script but are assigned different parts and blocked from even seeing anyone else’s lines. The fertilized egg possesses the first copy of the script; as it divides repeatedly into the 10 trillion cells of the human body, the cells assign themselves to the different roles they will play throughout an individual’s lifetime.
How does this assignment process work? The answer, researchers are finding, is that a second layer of information is embedded in the special proteins that package the DNA of the genome. This second layer, known as the epigenome, controls access to the genes, allowing each cell type to activate its own special genes but blocking off most of the rest. A person has one genome but many epigenomes. And the epigenome is involved not just in defining what genes are accessible in each type of cell, but also in controlling when the accessible genes may be activated.
http://www.nytimes.com/2009/02/24/science/24chromatin.html
A cell type is a classification used to distinguish between morphologically or phenotypically distinct cell forms within a species. A multicellular organism may contain a number of widely differing and specialised cell types, such as muscle cells and skin cells in humans, that differ both in appearance and function yet are genetically identical. Cells are able to be of the same genotype, but different cell type due to the differential regulation of the genes they contain. Classification of a specific cell typ
http://en.wikipedia.org/wiki/Cell_type
In multicellular organisms, gene regulation drives the processes of cellular differentiation and morphogenesis, leading to the creation of different cell types that possess different gene expression profiles, and hence produce different proteins/have different ultrastructures that suit them to their functions (though they all possess the genotype, which follows the same genome sequence). ...(Repressors bind to the Operator, coding sequences on the DNA strand that are close to or overlapping the promoter region, impeding RNA polymerase's progress along the strand, thus impeding the expression of the gene)
http://en.wikipedia.org/wiki/Regulation_of_gene_expression
All human cells share the same genetic information encoded by genomic DNA sequences, regardless of the cells’ type. However, cells exhibit dramatically diverse phenotypes. In eukaryotic cells, genomic DNA is modulated by numerous chemical modifications, thus adding an extra layer of information to the genome sequence. These modifications enable genomic DNA to encode a vast and complex program of gene regulation, giving rise to diverse protein expression patterns and subsequent tissue-specific phenotypic diversity. Discovering functional elements and understanding how diverse modifications regulate these elements are central challenges to elucidate global gene regulation in humans.
http://nar.oxfordjournals.org/content/early/2013/08/14/nar.gkt712.full
A transcriptional activator is a protein that increases gene transcription of a gene or set of genes.
http://en.wikipedia.org/wiki/Activator_(genetics)
In molecular biology, an inducer is a molecule that starts gene expression. An inducer can bind to repressors or activators.
Inducers function by disabling repressors. The gene is expressed because an inducer binds to the repressor. The binding of the inducer to the repressor prevents the repressor from binding to the operator. RNA polymerase can then begin to transcribe operon genes.
Inducers also function by binding to activators. Activators generally bind poorly to activator DNA sequences unless an inducer is present. Activator binds to an inducer and the complex binds to the activation sequence and activates target gene.[1] Removing the inducer stops transcription.
http://en.wikipedia.org/wiki/Inducer_(biology)
In molecular genetics, a repressor is a DNA- or RNA-binding protein that inhibits the expression of one or more genes by binding to the operator. A DNA-binding repressor blocks the attachment of RNA polymerase to the promoter, thus preventing transcription of the genes into messenger RNA. An RNA-binding repressor binds to the mRNA and prevents translation of the mRNA into protein. This blocking of expression is called repression...
If an inducer, a molecule that initiates the gene expression, is present, then it can interact with the repressor protein and detach it from the operator. RNA polymerase then can transcribe the message (expressing the gene). A corepressor is a molecule that can bind to repressor and make it bind to the operator tightly, which decreases transcription. A repressor that binds with a corepressor is termed an aporepressor or inactive repressor. One type of aporepressor is the trp repressor, an important metabolic protein in bacteria.
The above mechanism of repression is a type of a feedback mechanism because it only allows transcription to occur if a certain condition is present: the presence of specific inducer(s). Within the Eukaryotic genome are regions of DNA known as silencers. These DNA sequences bind to repressors to partially or fully repress the expression of a gene. Silencers can be located several bases upstream or downstream from the actual promoter of the gene. Repressors can also have two binding sites: one for the silencer region and one for the promoter. This causes chromosome looping always the promoter region and the silencer region to come to close proximity.
http://en.wikipedia.org/wiki/Repressor
How can Cells have the same DNA but different functions?
Answer: Although all calls have the DNA only some parts of it are turned on different cells (this is called gene expression). Which genes of the DNA are turned on determine the functions and kinds of cell. Even in the same kinds of cell genes can be differently expessed depending on what the cell needs to do at the time. If you have a drink of alcohol for instance a whole bunch of genes turn on in the liver to detoxify the alcohol. When you have meal hundreds of genes in the stomach, intestine and blood stream kick into action to digest the food and send it away for storage or immediate use. Cells have many things that regulate gene expression some things turn genes off or down others turn them up or on. There are factors turn on whole cascades of genes.
Medicare In
http://wiki.answers.com/Q/How_can_Cells_have_the_same_DNA_but_different_functions
http://en.wikipedia.org/wiki/List_of_distinct_cell_types_in_the_adult_human_body
Comparative Embryology - Developmental Biology - NCBI
https://www.ncbi.nlm.nih.gov/books/NBK9974/